Ontogeny and population biology of a sex-limited colour polymorphism

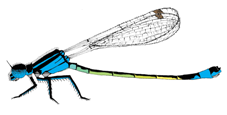
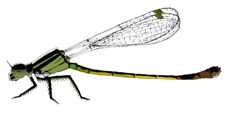
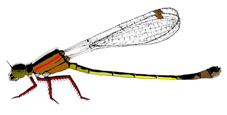
I. elegans male and female morphs. From top to bottom: male, Androchrome female, Infuscans female, Infuscans-obsoleta female.
For my PhD thesis I studied a number of populations of a colour polymorphic damselfly (Ischnura elegans) over several years, looking at frequency changes over time, morphological differences between the sexes and the morphs, differences in growth rate and development time, differences in fecundity between the morphs, and genetic differentiation between populations. Results indicated that the morphs are subject to negative frequency-dependent selection via male mating harassment, and that the differences between the morphs are part of their identity as alternative adaptive strategies.
I. elegans is a small annual damselfly with three female colour morphs: Androchrome (considered a male mimic), Infuscans, and Infuscans-obsoleta. Males are monomorphic. I calculated how the fecundity of each morph varied according to the frequencies of itself and the other morphs in our study populations and found evidence of negative frequency-dependent selection. Empirically estimated selection coefficients were used in a dynamic population genetic model with environmental variation which predicted coexistence of the three morphs, so this suggests that negative frequency-dependence on fecundity mediated by male harassment of the most common morph is sufficient to explain coexistence of the morphs.
An investigation of morph frequency changes in natural populations found that over 4 years, Androchrome frequency declined and Infuscans frequency increased, and that the pattern of morph frequency changes differed between new and old populations. I also found that males emerge earlier than females in the laboratory, and that females had a higher growth rate than males and were larger in the adult stage, indicating that sexual size dimorphism (SSD) is produced in I. elegans by a combination of development time and development rate acting in concert. The degree of sexual dimorphism in size and shape varied between populations, which could be a result of differential sensitivity of the sexes to different abiotic or biotic environmental conditions between populations. The female morphs also differed in development time and growth rate, and these differences cancel each other out with respect to final adult size (as compared to SSD, where they reinforce each other).
Collaborators: Erik Svensson, Lund University, Tom Gosden, University of Queensland, Roger Härdling, and Staffan Bensch, Lund University
